Introduction
As we approach Spring 2021, we are beginning to come out of the most devastating phase of the COVID-19 pandemic. In the United States, we’re averaging 71,718 new cases per week, with a 7-day average death rate of 1,907 per day. The US has recorded over 28 million cases of COVID-19 and more than 500,000 deaths since the pandemic began in March 2020. In addition to the devastating death and infection rates, pandemic fatigue is significantly impacting our country after a full year of restrictions. There is an intense focus on a COVID-19 vaccination program that can potentially eradicate the pandemic.
As we approach Spring 2021, we are beginning to come out of the most devastating phase of the COVID-19 pandemic. In the United States, we’re averaging 71,718 new cases per week, with a 7-day average death rate of 1,907 per day. The US has recorded over 28 million cases of COVID-19 and more than 500,000 deaths since the pandemic began in March 2020. In addition to the devastating death and infection rates, pandemic fatigue is significantly impacting our country after a full year of restrictions. There is an intense focus on a COVID-19 vaccination program that can potentially eradicate the pandemic.
There have been many obstacles to overcome while managing this vaccine delivery operation. For example, only 61.3 million doses have been administered thus far. Furthermore, only 17.9 million people or 5.5% of the total US population have been fully vaccinated. Scientists suggest that over 70% of the population needs to be vaccinated in order to achieve herd immunity. Vaccinating over 230 million people is an enormous and complex task. Fortunately, resourceful healthcare providers and IT vendors are addressing some of the challenges inherent to the COVID-19 vaccination rollout.
What follows is a practical road map to guide healthcare providers in understanding the requirements to implement a successful COVID-19 vaccination program.
UNDERSTANDING VACCINE DISTRIBUTION AND ADMINISTRATION
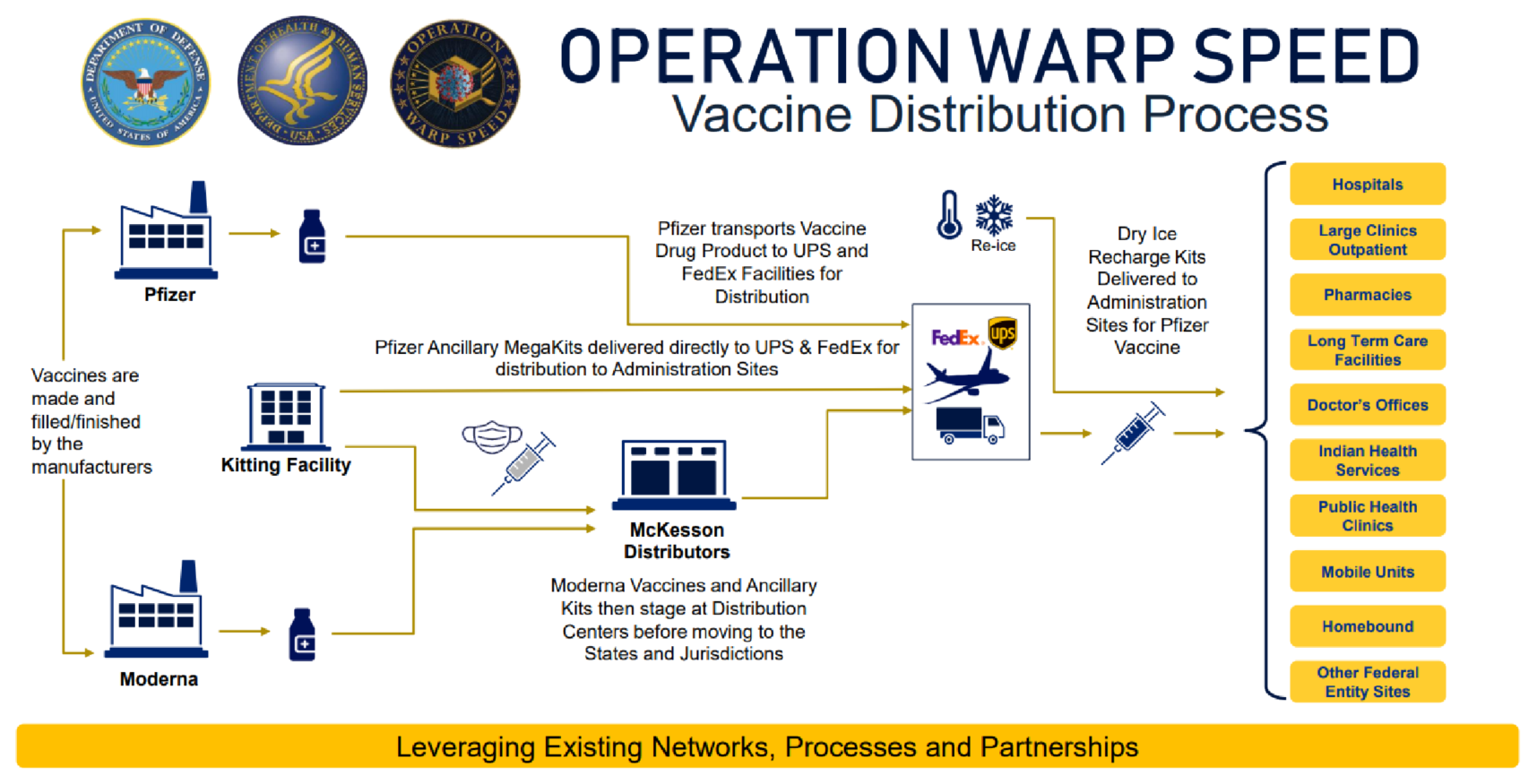
Operation Warp Speed Distribution Process
1. Authorization
By utilizing the Federal Food and Drug Administration’s (FDA’s) Emergency Use Authorization (EUA) to fast-track vaccines for public health emergencies, two drugs were approved for distribution in December 2020. These drugs are manufactured by Pfizer-BioNTech and Moderna. A few additional drug manufacturers are expected to receive EUA this spring.
2. Prioritization
The Centers for Disease Control and Prevention (CDC) recommended that the first phase of the COVID-19 vaccination program targets healthcare professionals and residents of long-term care facilities. However, state governors and local jurisdictions have some leeway in determining who will receive the vaccinations first and how best to manage the administration process. The CDC has since broadened their recommended vaccination list to include individuals over age 65, and other essential workers.
3. Allocation
Vaccine allocations for each state are based on the population of adult residents. The US currently has 400 million vaccine doses ordered for delivery, which is enough for 200 million recipients. President Biden recently announced that another 200 million doses will arrive this summer. By the fall, 300 million Americans should be fully vaccinated. As other manufacturers receive EUA, new drugs will be added to the distribution network with their delivery requirements. For healthcare providers, organizing efficient vaccine administration based on this allocation process is challenging. After an initial error-prone distribution process, states now consistently know their weekly vaccine allotment for consecutive 3-week periods. However, that allotment is adjusted every 3-weeks, creating new challenges.
4. Distribution
Vaccine distribution follows the processes outlined below:
- Vaccines are created at pharmaceutical manufacturing plants.
- Moderna vaccines are shipped to FDA-registered kitting facilities, where they are staged prior to shipment by UPS and FedEx.
- Pfizer vaccines are shipped directly to UPS and FedEx distribution facilities.
- UPS and FedEx ship vaccines directly to provider facilities
The Process from Distribution to Patient Administration
Participating providers are required to sign a CDC COVID-19 Vaccination Program Provider Agreement and are responsible for meeting the requirements below:
- Prioritization: Vaccines must be administered in accordance with prioritization groups determined by public health authorities.
- Record-keeping: The following information must be included in the patient’s record:
- Administration address, company, and date.
- Healthcare provider’s name and suffix.
- Vaccine provider’s address, if different.
- Recipient name, ID, sex, date of birth, and address.
- Vaccine administration site and route of administration.
- CVX (product), MVX (manufacturer), and NDC (national drug code).
- Lot and dose number, unit of use or sale, and vaccine expiration date.
- Safe Immunization Practices: The CDC has issued interim guidance for healthcare personnel to ensure safe vaccine administration during a pandemic.
- Vaccine Storage and Handling: Vaccines must be stored at specific freezer temperatures, and handled properly until they are administered to maintain the cold chain.
- Reporting to VAERS: Healthcare professionals are required to report certain adverse events by using the Vaccine Adverse Event Reporting System (VAERS). The US uses this early warning system to detect possible safety problems with vaccines.
Data and Reporting Requirements
- Vaccine Inventory: COVID-19 vaccine providers must report inventory daily into VaccineFinder, a free online service where users can search for vaccination sites.
- Vaccine Administration Data: Providers must document details of their vaccine administration in their medical records systems within 24 hours of administration. They also must report their data to the Immunization Information System (IIS) within 72 hours of dose administration
Challenges in Managing a Covid-19 Vaccination Program
In this effort to vaccinate 300 million Americans, one can see how vaccine administration presents complex challenges for healthcare organizations.
Supply Chain Management
With the CDC and states frequently changing prioritization requirements and allocation guidelines, many healthcare providers are struggling to predict when they will receive their vaccines and how many doses will be provided. Additionally, new vaccine administration sites are being established, such as pharmacies, grocery stores, sports arenas, and FEMA constructed vaccination tents. Reorganizing to address the scale of this project has resulted in frustration and inefficiency for both providers and patients. In New York, for example, 23,000 people had their first vaccine appointments rescheduled due to a lack of supplies. In North Carolina, small healthcare providers had their allocations redirected to mass vaccination sites. Thus, it is critical that supply chain issues are addressed quickly.
Storage and Waste Management
Since both vaccines must be stored in freezers, and the Pfizer vaccine requires an extremely low temperature, any facility administering vaccines must have proper storage equipment. Further complicating the matter is the requirement that both vaccines must be given in two doses, and at different time intervals. Waste management challenges arise because vaccines cannot be refrozen. Pfizer vials must also be mixed with a saline diluent before delivery. Once a vial is punctured for either manufacturer, the timeline for expiration is very short.
| Vaccine Storage, Prep & Delivery | Pfizer Vaccine | Moderna Vaccine |
|---|---|---|
| Frozen Storage | Between -112°F and -76°F | Between 36°F and 46°F |
| Diluent Required | YES, 0.9% Sodium Chloride | NO |
| Thawed Storage (no puncture) | Up to 120-hrs (5-days) | Up to 720-hrs (30-Days) |
| Thawed Storage (punctured vial) | 6-hours | 12-hours |
| Vaccine Dose Deliver Intervals | Minimum 21-days | Minimum 28-days |
Vaccine Administration and Workflow Management
Healthcare organizations must address numerous interconnected challenges to successfully build a COVID-19 vaccination program:
- Massive Scale: The goal is to fully vaccinate 300 million Americans by Fall 2021.
- Inconsistencies in Vaccine Supply Chain: Vaccine distribution, allocation, and priorities frequently change at the federal, state, and local levels.
- Logistics and Workflow: Administering COVID-19 vaccines on a large scale with all the supply chain, safety protocols, and data reporting requirements has led healthcare organizations to collaborate with community stakeholders to effectively manage their programs.
- Overlap with Common Flu: The symptoms of the flu and COVID-19 have considerable overlap. Beyond the stress of giving out millions of COVID-19 vaccines, there is a need to rapidly confirm and track flu diagnoses.
- Limited Skilled Human Resource Pool: Considering the project scope and timelines, providers must recruit and train additional workers.
- Billing and Reimbursement: COVID-19 vaccines are free for patients, but healthcare providers need to submit new billing codes to get reimbursed for vaccine administration services. With EUA, the coding and billing mechanisms require extra steps to ensure vaccination sites are paid in full.
- Data Collection and Reporting: Tracking inventory, reporting CDC required vaccine administration data, and submitting CPT and administrative billing codes puts a strain on resources. Agile healthcare IT is required to address patient throughput challenges. For example, due to the vaccine’s EUA status, pharmaceutical companies can ship drug vials without 2-D barcode IDs. This makes tracking and reporting much more difficult. With additional vaccines pending approval, the need for technology that optimizes workflows is more important than ever.
Strategies to Manage Covid-19 Vaccination Programs
There is a myriad of challenges with the COVID-19 vaccination rollout. Fortunately, several innovative solutions have been created to address the urgent need for this critical program. Several essential planning elements are outlined below, and they are based on lessons learned from healthcare organizations that have successfully initiated COVID-19 vaccination programs.
Establish a Planning and Coordination Team
When considering a COVID-19 vaccination program, a broad spectrum team of internal leadership should be assembled. It should include executive, administrative, clinical, IT, legal, PR, and risk management experts. It is also wise to consider representation from a local public health authority and to identify a state governance representative.
Manage Logistics and Patient Throughput
- Workspace: Establishing a workflow that is COVID-19 safe and an efficient physical workspace is the first consideration in planning a vaccination program. Many organizations are converting meeting rooms, outpatient clinics, and outdoor lots, with enough space to safely staff multiple vaccination stations. If workspace options are limited, organizations can collaborate with community businesses to convert meeting spaces and parking lots into vaccination centers.
- Staffing: The Department of Health and Human Services (HHS) recently amended its Public Readiness and Emergency Preparedness Act (PREP). The new amendment allows active licensed and certified healthcare professionals to work outside their home states when there is a resource shortage elsewhere. The declaration also authorizes healthcare professionals who retired within the past five years to be reactivated to assist in state vaccination programs. All qualified professionals are required to take a CDC COVID-19 Vaccine training course.
- Patient Throughput: Vaccination workflow can be organized into five categories:
- 1. Patient intake and registration
- 2. Vaccine prep and admixture by pharmacists and lab techs.
- 3. Vaccine administration by clinicians.
- 4. Adverse event monitoring by clinicians.
- 5. Data collection and reporting by a medical scribe.
Clinicians are most effective when paired with medical scribes (in a 1:1 ratio). Each well-trained clinical/scribe team (supported by a pharmacy) can quickly become proficient and process in excess of 100-patient vaccinations per team, per hour!
Leverage Technology
Electronic Health Record Optimization
With the many time-sensitive data collection and reporting requirements placed on all CDC-certified COVID-19 vaccination programs, leveraging existing electronic medical records and ancillary software applications is essential to success. Optimizing EHR systems can be very effective in improving productivity, reducing healthcare provider fatigue, and improving reporting capabilities.
2-D Barcode Technology
Implementing innovative 2-D barcode technology applications can automate data collection, decrease stress, reduce administrative errors, and thereby increase patient throughput. Enhanced barcode applications have the added benefit of automating critical inventory tasks, which allows pharmacy departments to mitigate drug storage and waste management issues. Select healthcare IT vendors are leveraging 2-D barcode functionality to create automated workarounds for EUA vaccines that are shipped without barcodes. To do so, they are incorporating customizable barcode generator tools. Some of these barcode applications even capture 2-series vaccination CPT billing and administrative codes to ensure that vaccine programs are reimbursed appropriately for their services.
Health System Significantly Enhances Vaccination Throughput
EHR Barcode Reader was recently installed at a clinic in South Florida. Pre-installation, the clinic staff were only vaccinating at a rate of ~95 patients per day. After implementing our barcode solution, along with suggested workflow adjustments, the clinic was able to increase its vaccination rate to over 400 people per day with zero waste. This clinic is now aiming to expand its hours with a goal of vaccinating 1,000 patients a day.
Conclusion
After a year of struggling to contain one of the world’s worst pandemics, we are at a crossroads in the history of the disease. We have the ability to defeat COVID-19 by being diligent with safety precautions, incorporating proven workflow processes, and leveraging technology to assist in deploying successful vaccination programs. Important lessons have been learned from vaccine program pioneers. We now have an effective road map to guide healthcare providers as they address the challenges at hand.
Tools That Can Help
Keena Healthcare Technology recognizes the importance of leveraging technology to streamline workflow and improve patient throughput. We would like to highlight a few solutions to help manage your COVID-19 vaccination programs more efficiently.
Barcode Reader Technology
Keena’s EHR Barcode Reader application leverages 2-D barcode technology to significantly reduce the amount of time spent administering and managing vaccinations. It improves patient throughput by automating the process of capturing CDC-required vaccine and administrative data. Our development team has added the following custom tools to address the EUA barcode data limitation:
- Customizable Vaccine Barcode Creator: This tool generates custom barcodes to capture NDC, lot, and expiration date.
- Vaccine Series Manufacturer and Administration Data Capture: This tool automatically captures the vaccine manufacturer, series number, and date. It also accurately reports on Series 1 vaccine administrations to qualify for Series 2 shipments.
- CPT Billing and Administration Code Capture: This tool simplifies the recording of Series-1 and Series-2 vaccine CPT billing and administrative codes. It ensures that appropriate reimbursement for vaccine services is given to healthcare facilities.
Automated Chart Export Tools
Keena’s Chart2PDF tool makes exporting patient records easy and efficient. It provides a quick conversion of patient charts to PDF format, which saves time and improves workflow. Chart2PDF can instantly save, export, print, and scan every document in a patient’s history, including COVID-19 vaccination records.
EHR Optimization, Conversions, Data Archival and Custom Reporting Services
At Keena Healthcare, our consultants are experts in leveraging existing electronic medical records and ancillary software applications to optimize time-sensitive data collection and reporting for state and federal regulatory bodies. Continual assessment of the productivity and capabilities of such systems is essential for mass vaccination programs.
InteleFiler
Utilize artificial intelligence and machine learning to capture critical data from fax and paper COVID results typically processed manually with an enormous amount of staff clicking, speeding up the time that critical information is sent to the patient chart and provider review.